On Feb. 27, 2014, First Lady Michelle Obama, Secretary of Health and Human Services Kathleen Sebelius and Food and Drug Administration (FDA) Commissioner Margaret Hamburg announced two proposed rules that would revise the appearance and contents of Nutrition Facts labels that are required to be printed on roughly 700,000 food and beverage products.
According to a White House press release, the proposed changes to the required labeling “are intended to reflect the latest scientific information about the link between diet and chronic diseases such as obesity and heart disease.” If adopted, the proposed rules would change the label design, the mandatory contents of nutritional information on the label and the determination of serving sizes of certain products. The FDA states that these changes are designed to help consumers “make informed food choices and maintain healthy dietary practices.”
Comments on the proposed rules must be received by June 2, 2014. According to the FDA, manufacturers would have two years after the effective date of the accepted changes to comply with any final requirements.
Changes to Label Design
The key changes to the label design include making the calorie and serving size information more prominent through use of a larger, bolder font and moving the “Percent Daily Value” to the left of the label. In addition, the “servings per container” would appear in a more pronounced type. An image of the proposed label design appears below. According to the FDA, the proposed changes to the label design “emphasize parts of the label that are important in addressing current public health concerns such as obesity, diabetes, and cardiovascular disease.”
Label Formats
Original vs. proposed
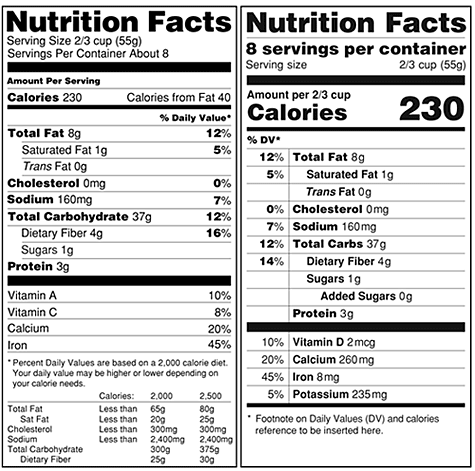
Revisions to Label Contents
If the proposed changes are approved, food labels would no longer be required to contain information about a serving’s number of calories from fat and the percentage daily value of vitamins A and C. Facts informing the consumer of the number of grams of “added sugars” and the percentage of daily value of vitamin D and potassium would be added. The FDA asserts that vitamin D and potassium should be added to the label as they are “new ‘nutrients of public health significance.’ ”
Modifications to Serving and Package Size Information
The recommended changes to the serving size requirements would better “reflect how people eat and drink today, which has changed since serving sizes were first established 20 years ago,” according to the FDA. The planned variations would require that packaged foods and drinks that are routinely eaten in one sitting be labeled as a single serving. Similarly, the calorie and nutrient information must be declared for the entire contents of the package. An example of this change is that a 20-ounce bottle of soda would be labeled as one serving and not multiple servings.
In addition, the suggested changes to the labeling would require certain larger packages that could be consumed in a single sitting or at multiple times to contain “dual column” labels that provide both “per serving” and “per package” information. The FDA cites 24-ounce sodas and a pint of ice cream as examples that would be impacted by this new requirement.
Additional information about the proposed rules can be found at www.fda.gov.