RELATED: End of an Era: White House Ends Many Federal COVID-19 Vaccine Requirements (May 3, 2023)
On Jan. 13, 2022, the U.S. Supreme Court permitted the Centers for Medicare & Medicaid Services (CMS) to enforce its interim final rule requiring many Medicare- and Medicaid-certified providers and suppliers to vaccinate their staff for COVID-19. Based on this decision and added guidance issued by CMS, covered providers are now subject to three different key compliance deadlines, depending on their location.
Specifically, in final guidance memoranda released Jan. 14 and Jan. 20, 2022, CMS provided separate deadlines for providers in the 24 states subject to the injunction lifted by the Supreme Court plus Texas (which was subject to a separate injunction). These deadlines differ from the deadlines previously given to providers within the other 25 states and the District of Columbia that were not subject to the earlier lower court injunction — all of which have an initial deadline of Thursday, Jan. 27, 2022.
As enforcement begins, providers face three different sets of deadlines and differing category-specific guidance (and related risks).
What are the different deadline groupings by state?
For providers and suppliers subject to Medicare and Medicaid Conditions of Participation, Conditions for Coverage or Requirements for Participation (see McGuireWoods’ Nov. 5, 2021, alert for category analysis), there are three groupings of deadlines based on location.
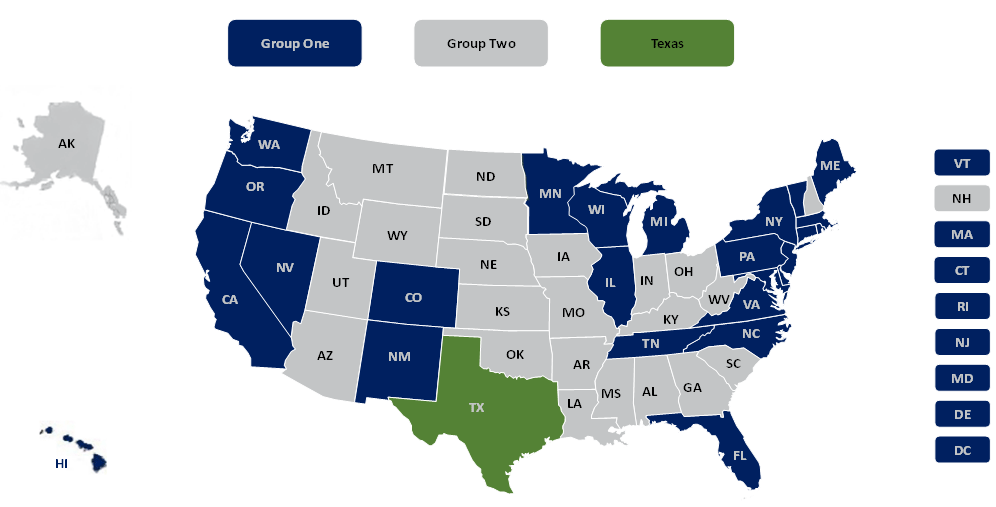
- Group 1: California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Hawaii, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Jersey, New Mexico, New York, North Carolina, Oregon, Pennsylvania, Rhode Island, Tennessee, Vermont, Virginia, Washington and Wisconsin
- Group 2: Alabama, Alaska, Arizona, Arkansas, Georgia, Idaho, Indiana, Iowa, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Montana, Nebraska, New Hampshire, North Dakota, Ohio, Oklahoma, South Carolina, South Dakota, Utah, West Virginia and Wyoming
- Texas
What are the deadlines for each state grouping?
There are two primary deadlines for each grouping under the vaccination rule. As outlined in its guidance to surveyors and in updated FAQs, CMS modified its original regulatory compliance dates to fall 30 days and 60 days after the issuance of each of the three guidance memos for Group 1, Group 2 and Texas, which are as follows:
| Grouping | Phase 1 Deadline | Phase 2 Deadline |
| Group 1 | Jan. 27, 2022 | Feb. 28, 2022 |
| Group 2 | Feb. 14, 2022 | March 15, 2022 |
| Texas | Feb. 22, 2022 | March 21, 2022 |
What does the vaccination rule require for each of the phase deadlines?
Phase 1
By the Phase 1 deadline:
- The facility must develop and implement policies and procedures for ensuring all facility staff (broadly defined to also include independent contractors, volunteers, etc. — see McGuireWoods’ November 5, 2021 alert) are vaccinated for COVID-19, regardless of clinical responsibility or level of patient or resident contact.
- The facility’s covered staff must receive at least one dose of a COVID-19 vaccine, excluding federally recognized exemptions for religious belief and disability and delayed dosing recommended by the Centers for Disease Control and Prevention (CDC).
Facilities without 100 percent staff compliance by the Phase 1 deadline face written notice of noncompliance by surveyors. However, CMS has instructed surveyors that if a facility has achieved 80 percent vaccination compliance and has a plan to achieve a 100 percent staff vaccination rate within 60 days, the facility should not be subject to additional enforcement action. Facilities that do not meet these parameters could face plans of correction, civil monetary penalties, denial of payment or termination, depending on the severity of the deficiency and type of facility.
Phase 2
By the Phase 2 deadline, the facility must ensure that 100 percent of its staff received the required doses to complete the COVID-19 vaccine series (i.e., one dose of a single-dose vaccine or all doses of a multiple-dose vaccine series). Again, federally recognized exemptions and CDC-delayed dosing recommendations do not count against the facility.
Facilities without 100 percent of staff completing the COVID-19 vaccine series face noncompliance in the same manner as discussed above. However, CMS instructed surveyors that if 90 percent of a facility’s staff is fully vaccinated, and the facility has a plan to achieve a 100 percent staff vaccination rate within 30 days, then the facility would not be subject to additional enforcement action. Facilities that do not meet these parameters could be subject to additional enforcement actions depending on the severity of the deficiency and type of facility.
Who is charged with enforcing the CMS interim vaccination rule?
The CMS guidance memoranda provide that federal, state, accreditation organization (e.g., Accreditation Association for Ambulatory Health Care, The Joint Commission, National Dialysis Accreditation Commission), and “CMS-contracted” surveyors will evaluate compliance with the vaccination rule during initial certifications, standard recertifications or reaccreditations, and complaint surveys.
This enforcement mechanism may create federalism issues and challenges, since in many states CMS works with state regulators to act as Medicare surveyors. Some of these states, however, have leaders who have promised not to enforce vaccine mandates — which may create ambiguity for some providers.
How will surveyors determine the punishment for noncompliance?
CMS has stated its “primary goal is to bring health care facilities into compliance.” To that end, CMS has instructed surveyors to determine the level of deficiency with respect to the facility’s noncompliance, based on the criteria below, and then apply certain corrective action. For example, a facility receiving a standard-level citation could clear the citation by obtaining full vaccination or could provide a schedule for staff vaccinations while the citation would remain. A determination of “immediate jeopardy” will require a larger plan of correction and likely an accelerated schedule for penalties.
- Standard Level
- Did not meet the 100 percent staff vaccination rate standard, but is making good faith efforts toward vaccine compliance; or
- 100 percent of staff are vaccinated, and all new staff have received at least one dose, but the facility did not develop or implement one or more components of the policies and procedures.
- 21–39 percent of the facility’s staff remain unvaccinated, creating a likelihood of serious harm; or
- the facility did not meet the 100 percent staff vaccination rate, and it did not develop or implement one or more components of the policies and procedures.
- 40 percent or more of the facility’s staff remain unvaccinated, creating a likelihood of serious harm; or
- the facility did not meet the 100 percent staff vaccination rate, the surveyor observes noncompliant infection control practices by staff (e.g., staff failed to properly use personal protective equipment) and the facility did not develop or implement one or more components of the policies and procedures (e.g., related to tracking staff vaccinations, documenting medical and religious exemptions).
Note: The example above is for an end-stage renal disease facility, but most facilities are similar.
CMS also noted that surveyors or the agency may adjust the level of citation and/or enforcement action if good faith efforts were taken to comply by demonstrating prior to the survey that the facility had limited access to vaccines or took aggressive steps to have all staff vaccines.
Is there additional provider-specific guidance for compliance?
Yes. In addition to the survey guidance linked above, the guidance memoranda include specific, tailored guidance for each type of covered provider in 14 separate attachments.
- Long Term Care and Skilled Nursing Facilities (Group One | Group Two | Texas)
- Ambulatory Surgery Centers (Group One | Group Two | Texas)
- Hospice (Group One | Group Two | Texas)
- Hospitals (Group One | Group Two | Texas)
- Psychiatric Residential Treatment Facilities (Group One | Group Two | Texas)
- Intermediate Care Facilities for Individuals with Intellectual Disabilities (Group One | Group Two | Texas)
- Home Health Agencies (Group One | Group Two | Texas)
- Comprehensive Outpatient Rehabilitation Facilities (Group One | Group Two | Texas)
- Critical Access Hospitals (Group One | Group Two | Texas)
- Outpatient Physical Therapy (Group One | Group Two | Texas)
- Community Mental Health Centers (Group One | Group Two | Texas)
- Home Infusion Therapy (Group One | Group Two | Texas )
- Rural Health Centers / Federally Qualified Health Clinics (Group One | Group Two | Texas)
- End-Stage Rental Disease Facilities (Group One | Group Two | Texas)
For questions about the vaccination rule, the import of the Supreme Court’s decisions, or assistance developing or updating policies and materials for implementation, please contact the authors of this article, your McGuireWoods contact, or a member of the firm’s healthcare, labor and employment, appeals and issues or COVID-19 response teams.